The views and statements In this particular web site are All those in the authors and don't always mirror These of BRG. This site is predicated on individual expertise and reviews of data publicly out there or recognized in other database queries.
“We partnered with Kellerman Consulting very early on from the SQF certification procedure, and by depending on their know-how and methodical method of gathering, compiling, and Arranging the demanded documentation, we were being effective in getting our certification.
KNORS workforce of pharma industry experts rationally design good quality management techniques and assist to implement by way of trainings.
Hence, all supplier qualification actions contribute to the general aims of medicinal product or service basic safety, effectiveness and compliance.
Our GMP Certification consultants, with their loaded and various knowledge in putting together state with the art producing amenities conceptualizing your desire project according to spending budget and laws.
This is often what an average course of action seems like: The GMP marketing consultant initially checks the business-distinct documentation and produces a gap Assessment system. According to this hole Assessment system, the Assessment on-site will be done.
The pharmaceutical discipline is stuffed with private information, from affected individual documents to trial final results to patented get the job done; it is completely vital to shield this knowledge read more and IP from any cybersecurity danger.
World wide source chain disruption and API scarcity will power the market to diversify sourcing destinations and build new provider networks – all even though furnishing authentic-time, full-solution visibility and traceability.
Crank out and disseminate evidence that answers crucial medical, regulatory and business questions, enabling you to push smarter conclusions and satisfy your stakeholder requires with self-confidence.
Our experts performs third party evaluation and gap Examination using a quantified Device to evaluate the corporate’s state of regulatory compliance. We enable the businesses in coming up with CAPA for deficiencies elevated by regulatory inspections.
A group of everlasting GMP consultants, specialized in various fields of competence, supports you in your responsibilities and issues with experience and foresight.
- Joining our IGAP system/ Audit report library is free for suppliers/manufacture. Our seasoned and certified auditor will perform whole site audit of provider masking big range of molecules/products (Otherwise now executed) without charge to provider and comprehensive audit report shall be prepared.
PJC Pharma Consulting presents a shopper-concentrated pharmaceutical consultancy and parenteral consultancy company, featuring complex read more guidance for item development, coaching, vital course of action assessments, audits and task management. With thirty many years’ working experience from the pharmacy/pharmaceutical market, we deal with a spectrum of jobs from generic portfolio advancement and registration to device advancement and registration, and new chemical entity enhancement to scientific phase.
Our gurus prepare all key regulatory submissions and provide comprehensive high quality Management evaluation for all trial-connected documentation, for example:
 Alana "Honey Boo Boo" Thompson Then & Now!
Alana "Honey Boo Boo" Thompson Then & Now! Val Kilmer Then & Now!
Val Kilmer Then & Now! Andrew Keegan Then & Now!
Andrew Keegan Then & Now!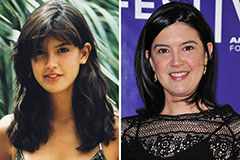 Phoebe Cates Then & Now!
Phoebe Cates Then & Now! Ricky Schroder Then & Now!
Ricky Schroder Then & Now!